Mathematical modeling of biological systems that are of interest in the pharmaceutical industry is a rapidly growing area. The use of mathematical models brings the promise of reducing the high costs and long times associated with the development of new drugs and models are gradually finding their way into the drug development routine.
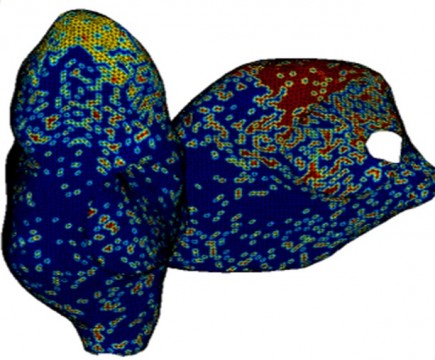
Computer models make it possible to relate the dynamics of the action potential propagation in realistic atrial geometries to drug effects at the single cell level. This in turn permits in silico reconstruction and investigation of phenomena like atrial flutter and fibrillation. FCC has developed a framework for modeling and simulation of electro-chemical activity in large scale cell networks. A geometric model of the canine atria has been constructed utilizing ultra sound imaging data and a realistic fiber structure and cell type distribution has also been incorporated.
The complete atrial tissue model consists of about 2.000.000 coupled nonlinear ordinary differential equations. To meet the computational demands of this model the developed modeling and simulation framework has been translated into a high performance computing setting first tested and executed on FCC’s internal computational servers and recently deployed onto Chalmers Centre for Computational Science and Engineering (C3SE) facilities. The simulation framework has been used to induce fibrillation and flutter like electro-dynamic activity in cell networks from simple sheets up to realistic atrial geometries. In addition, the effect of ion channel modulation on this behavior has been investigated. The simulations are in good accordance with in vivo observations, have great potential to provide insights into the underlying mechanisms of atrial fibrillation and flutter, and can serve as a tool for prediction of drug effects.
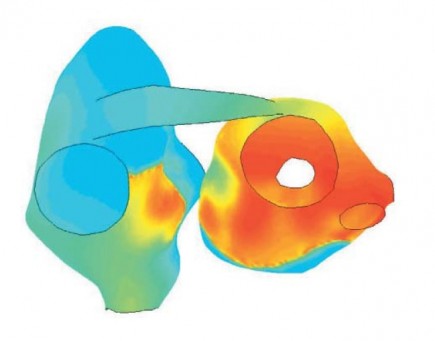
Finding suitable targets and drugs that modulate their activity appropriately is difficult. Some new anti-arrhythmic drugs are targeting multiple ion channels simultaneously. As the possible combinations of targets, modulation type, and relative strength of the effect on the different targets are overwhelmingly complex, such treatment strategies present an even more difficult problem. Predictive mathematical models may be particularly suited to identify useful multi-target drug profiles. The importance of foresight in choosing drug candidates is pivotal as very few compounds will eventually reach the market. Hence, an interesting area for future development of our atrial modeling efforts would be in silico investigations of different multi-target strategies.
Future
Finding suitable targets and drugs that modulate their activity appropriately is difficult. Some new anti-arrhythmic drugs are targeting multiple ion channels simultaneously. As the possible combinations of targets, modulation type, and relative strength of the effect on the different targets are overwhelmingly complex, such treatment strategies present an even more difficult problem. Predictive mathematical models may be particularly suited to identify useful multi-target drug profiles. The importance of foresight in choosing drug candidates is pivotal as very few compounds will eventually reach the market. Hence, an interesting area for future development of our atrial modeling efforts would be in silico investigations of different multi-target strategies.